-
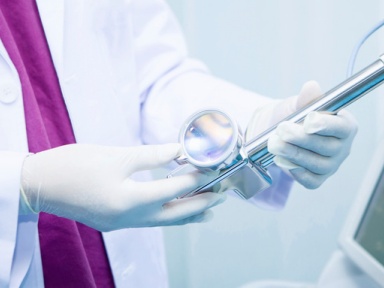
InnoCare Releases 2024 Results and Business Highlights
PharmaSources
March 27, 2025
InnoCare announced 2024 annual results, including financials, R&D progress in oncology and autoimmune diseases, and globalization efforts.
-
InnoCare Announces First Patient Dosed in the Phase III Registrational Trial of ICP-488 for the Treatment of Psoriasis in China
PharmaSources
March 20, 2025
InnoCare announced the first patient dosing in the Phase III trial of ICP - 488 for psoriasis. It showed good results in Phase II, aiming to meet unmet needs.
-
InnoCare Announces the Approval of Registrational Phase III Study of BCL2 Inhibitor ICP-248 in Combination with Orelabrutinib as First-Line Therapy for Treatment of CLL/SLL Patients in China
PharmaSources
February 18, 2025
InnoCare Pharma announced on Feb. 17, 2025 that CDE approved the Phase III trial of ICP - 248 combined with orelabrutinib for first - line CLL/SLL treatment in China.
-
InnoCare Announces First Subject Dosed in the Phase III Registrational Trial of TYK2 Inhibitor ICP-332 for the Treatment of Atopic Dermatitis in China
PharmaSources
November 15, 2024
InnoCare Announces First Subject Dosed in the Phase III Registrational Trial of TYK2 Inhibitor ICP-332 for the Treatment of Atopic Dermatitis in China
-
InnoCare Announces Phase II Study Results of TYK2 Inhibitor ICP-488 Meet Primary Endpoint in Psoriasis Patients
PharmaSources
October 10, 2024
InnoCare Pharma announced today that phase II clinical study results of novel TYK2 (Tyrosine Kinase 2) inhibitor ICP-488 met the primary endpoint in adult patients with moderate-to-severe plaque psoriasis.
-
InnoCare Announces Approval of Clinical Trial of BCL2 Inhibitor ICP-248 for Acute Myeloid Leukemia in China
PharmaSources
September 19, 2024
InnoCare Pharma announced today the approval of the Investigational New Drug (IND) to conduct the clinical trial of B-cell lymphoma-2 (BCL2) inhibitor ICP-248 in combination with azacitidine for acute myeloid leukemia (AML) in China.
-
Latest Data of InnoCare’s Orelabrutinib Presented at the European Society for Medical Oncology (ESMO) Congress 2024
PharmaSources
September 14, 2024
Latest data of InnoCare’s (HKEX: 09969; SSE: 688428) orelabrutinib were presented at the European Society for Medical Oncology (ESMO) Congress 2024.
-
InnoCare Releases 2024 Interim Results and Business Highlights
August 21, 2024
InnoCare Pharma announced the 2024 interim results and corporate update as of 30 June 2024.
-
British Journal of Cancer: Zurletrectinib Is a Next-Generation TRK Inhibitor With Strong Intracranial Activity Against NTRK Fusion-Positive Tumors With On-Target Resistance to First-generation Agents
July 31, 2024
Zurletrectinib is a next-generation TRK inhibitor with strong intracranial activity against NTRK fusion-positive tumors with on-target resistance to first-generation agents.
-
InnoCare Announces First Subject Dosed in Clinical Trial of TYK2 Inhibitor ICP-332 in the U.S.
July 24, 2024
Beijing, July 24, 2024 – InnoCare Pharma (HKEX: 09969; SSE: 688428), a leading biopharmaceutical company focusing on the treatment of cancer and autoimmune diseases


 InnoCare Pharma announced the 2024 interim results and corporate update as of 30 June 2024.
InnoCare Pharma announced the 2024 interim results and corporate update as of 30 June 2024. Zurletrectinib is a next-generation TRK inhibitor with strong intracranial activity against NTRK fusion-positive tumors with on-target resistance to first-generation agents.
Zurletrectinib is a next-generation TRK inhibitor with strong intracranial activity against NTRK fusion-positive tumors with on-target resistance to first-generation agents. Beijing, July 24, 2024 – InnoCare Pharma (HKEX: 09969; SSE: 688428), a leading biopharmaceutical company focusing on the treatment of cancer and autoimmune diseases
Beijing, July 24, 2024 – InnoCare Pharma (HKEX: 09969; SSE: 688428), a leading biopharmaceutical company focusing on the treatment of cancer and autoimmune diseases ALL
ALL Pharma?in?China
Pharma?in?China Pharma?Experts
Pharma?Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025








