July 04, 2025
Tag:
1 Preface
During the saponification process, sodium chloride is usually used as the salting-out agent, and the alkali used often contains sodium chloride as well. So, soap always contains a certain amount of chloride. The content of chloride has a significant impact on the microstructure of soap. If the chloride content is too high, it will cause the soap structure to become coarse and loose, increase the degree of cracking, and affect the storage and use of soap.
This method is applicable to the determination of chloride ion content greater than or higher than 0.1%. Soap is decomposed into fatty acids by acid, and the chloride ions in the solution are precipitated with silver nitrate. Finally, the excess silver nitrate is titrated with ammonium thiocyanate, and the chloride ions in the soap are detected through reverse titration. It has the advantages of simple operation, rapid titration and accurate results.
2 Instruments and reagents
2.1 Instruments
JH-T6 fully automatic potentiometric titrator, 10mL burette with composite silver electrode
2.2 Reagents
Nitric acid, ammonium thiocyanate titrant (0.1mol/L), silver nitrate titrant (0.1mol/L), grade one water.
3 Experimental Methods
3.1 Experimental Procedures
Weigh 5.00g of the soap sample and place it in a 250mL volumetric flask. Add 5mL of nitric acid and 25.0mL of silver nitrate (0.1mol/L). Place it in boiling water until the fatty acids are completely separated and a large amount of silver chloride precipitate accumulates. Cool it to room temperature with tap water and dilute it to the mark with water. Dare to filter, discard the initial 10mL, collect 200mL of filtrate, take 50mL, and use ammonium thiocyanate
Titrate (0.1mol/L) to the end point of the potential jump and record the end volume.
3.2 Instrument Parameters

4. Results and Discussion
4.1 Experimental Data
4.1.2 Determination of chloride ion Content in soap
|
Sample name |
Concentration of titrant (mol/L |
Sampling volume (g |
Titration volume (mL |
Chloride ion content (in. (Nacl meter) % |
RSD ( % ) |
|
Soap |
0.10 |
4.99983 |
4.230 |
0.9676 |
0.5776 |
|
4.306 |
0.9587 |
||||
|
4.317 |
0.9574 |
4.2 Calculation Formula
Among them:
C1 - Molar concentration of silver nitrate standard solution, mol/L;
C2 - Molar concentration of ammonium thiocyanate standard titration solution, mol/L;
V- Volume of ammonium thiocyanate standard solution consumed, mL;
0.0585 The millimolar mass of sodium chloride expressed in grams (g/mmol) in the test;
m- Mass of the sample, unit (g)
4.3 Titration Chromatogram
4.3.1 Determination of Chloride Ion Content in Soap:
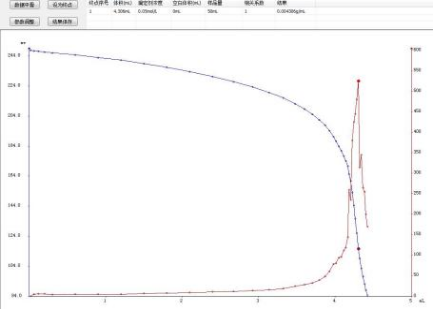
4.4 Conclusion
The JH-T6 fully automatic potentiometric titrator was used to determine the chloride ions in soap. The measurement results had good repeatability and could accurately locate the titration endpoint. The titration process could be automated, which was more convenient and faster than the traditional manual titration.
References
[1] QB/T 2623.6-2003 Test Method for Soap - Determination of Chloride Content in Soap - Titration method.[S]


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


