June 06, 2025
Tag:
1. Preface
Acid value refers to the milligram number of potassium hydroxide required to neutralize free fatty acids in 1g of oil. Generally, fresh and pure oil obtained from normal raw materials has a very low acid value, not exceeding 2 to 3, and the acid value of edible oil should not be higher than 5. ARA oil, as a nutritional fortifier, plays a significant role in preventing diabetes, cardiovascular diseases and other conditions. Therefore, its acid value is clearly regulated. In this paper, the acid value of ARA oil was determined by referring to the national standard GB 5009.229-2016 to judge whether it was qualified. This experiment avoided the subjective error caused by visual judgment and the data repeatability was good.
2 Instruments and equipment
2.1 Instruments
JH-T7 fully automatic potentiometric titrator, PH non-aqueous composite electrode.
2.2 Reagents
Sodium hydroxide standard solution (0.1 mol/L), anhydrous ether, isopropanol.
3 Experimental Methods
3.1 Experimental Procedures
Accurately weigh 20g of the sample (accurate to 0.00001g) and place it in the titration cup, then add 50mL of ether - isopropanol (1: 1) Mix the solution to ensure it covers the electrode, place it on the potentiometric titration table, start the stirring to ensure the sample is completely dissolved, insert the electrode and the titration head. After the potential stabilizes, titrate with a sodium hydroxide (0.100mol/L) standard solution to the potential jump point, record the volume of the sodium hydroxide standard titration solution consumed, and conduct a blank test simultaneously.
3.2 Parameter Settings
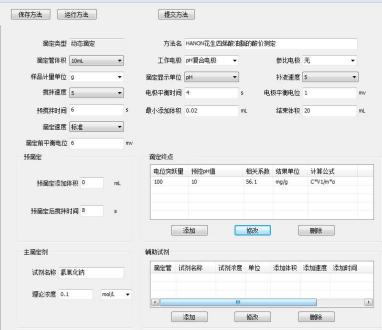
4 Results and Discussion
4.1 Experimental Results
|
Sample Name |
Sample Number |
Titration Solution Concentration (mol/L ) |
Sample Volume (g) | Titration Volume V1 (mL) | Blank Volume V0 (mL) | Acid Value (mg/g) |
Average value |
|
ARA Grease |
1 |
0.10115 |
15.1079 |
0.560 |
|
0.2028 |
0.2029 |
|
2 |
18.4124 |
0.680 |
0.2034 |
||||
|
3 |
18.0421 |
0.664 |
0.2025 |
Calculation formula:
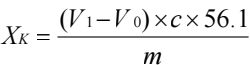
In the formula:
Xk is the acid valence, with the unit being milligrams per 100 grams (mg/100g).
V1 and V0 are respectively the volumes of the titrant consumed for titrating the sample and the blank.
56.1 is the molar mass of potassium hydroxide;
m is the mass of the sample
4.2 Atlas
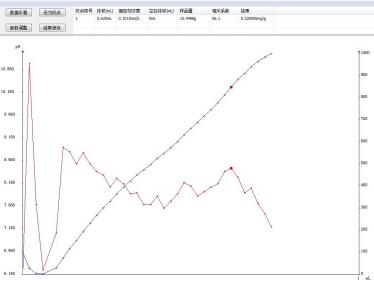
4.3 Conclusion
The determination of acid value in ARA oil by JH-T7 fully automatic potentiometric titrator has good repeatability, and the determination results are within its standard range (≤1mg/g). The T960 fully automatic potentiometric titrator fully meets the requirements for determining the acid value of oil products.
References
[1] GB 5009.229-2016 National Food Safety Standard - Determination of Acid Value in Foods [S]


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094


