May 14, 2025
Tag:
1. Preface
Vitamin B1 is a vitamin that the human body cannot synthesize on its own and must be obtained from external foods. A deficiency of vitamin B1 can lead to beriberi, for which it is necessary to obtain vitamin B1 from the outside. How to choose which foods to consume and the determination of vitamin B1 content play a crucial role. Meanwhile, for the pharmaceutical industry, the content of vitamin B1 produced is an important part of scientific research and production, and the determination of its content is also of great significance.
The potentiometric titration method was selected to determine the content of vitamin B1. It has a fast titration speed, good data repeatability, reduces the contact between personnel and reagents, improves safety and enhances the efficiency of content detection at the same time.
2 Instruments and reagents
2.1 Instruments

JH-T7 Fully automatic potentiometric titrator (non-water) PH composite electrode
10mL burette
2.2 Reagents
Perchloric acid titration (0.1mol/L), acetic anhydride (AR grade), glacial acetic acid (AR grade)
3 Experimental Methods
3.1 Experimental Procedures
Accurately weigh 0.12g of the sample, add 20mL of glacial acetic acid, slightly heat to dissolve, slightly cool, add 30mL of acetic anhydride, titrate with perchloric acid (0.1moL/L) to the emergent endpoint, and record the volume at the endpoint.
3.2 Instrument Parameters
|
Titration mode: |
Dynamic titration |
Stirring speed: |
5 |
|
Electrode equilibrium time: |
4s |
Pre-mixing time: |
5s |
|
Electrode equilibrium potential: |
1mv |
Titration rate: |
slow |
|
Minimum addition volume: |
0.02mL |
Pre-titrate and add volume: |
0.01mL |
|
End volume: |
10mL |
Pre-titration stirring time: |
8s |
|
Potential leap quantity: |
300 |
Pre-control the mv value: |
no |
4 Results and Discussion
4.1 Experimental Data
4.1.2 Determination of Vitamin B1 Content:
| Sample | Titration solution concentration (moL/L) | Sampling quantity (g) | Titration volume (mL) |
Glycine content (%) |
Average value (%) |
RSD ( % ) |
|
一 |
0.1044 |
0.10660 |
6.060 |
100.0630 |
100.05 |
0.4013 |
|
0.10176 |
5.807 |
100.4460 |
||||
|
0.10099 |
5.717 |
99.6432 |
||||
|
二 |
0.10954 |
6.237 |
100.2215 |
100.18 |
0.1546 |
|
|
0.10108 |
5.760 |
100.3033 |
||||
|
0.10133 |
5.757 |
100.0037 |
4.2 Calculation Formula
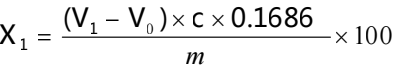
X1- Vitamin B1 content (%)
V1- The volume of perchloric acid titrant consumed by the sample, in mL; C- The actual concentration of perchloric acid titrant, mol/L;
V0- The volume of perchloric acid consumed by the blank, in mL;
The mass of vitamin B1 equivalent to 16.86 mL of 1.00mL of perchloric acid standard solution [c=0.1mol/L]. m- The mass of the sample, unit (g)
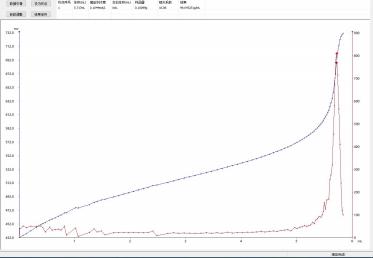
4.3 Titration Spectrum
4.3.1 Vitamin B1 content:
4.4 Conclusion
The content of vitamin B1 was determined by the JH-T7 fully automatic potentiometric titrator. The data repeatability was good, the operation was convenient and safe, and it met the requirements for vitamin B1 detection in the laboratory.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


