July 04, 2025
Tag:
1 Preface
Sodium sulfite is a small white or yellow granule, generally used as a reducing agent. In wastewater treatment, it can reduce toxic heavy metals and also has a bactericidal effect. Sodium sulfite is also used in the water treatment industry as a deoxidizer for boiler water, industrial water, as well as an antioxidant and preservative. The content of sodium sulfite in wastewater was determined by potentiometric titration.
2. Instruments and equipment
2.1 Instruments
JH-T6 fully automatic potentiometric titrator, platinum composite electrode
2.2 Reagents
Iodine solution (0.1mol/L), hydrochloric acid (1+ 1), sodium thiosulfate titrant (0.1mol/L)
3 Experimental Methods
3.1 Experimental Procedures
Weigh approximately 0.08g of the sample into a titration cup, and accurately add 25mL of iodine solution (0.1mol/L) and 2mL of salt using a pipette
Mix 1+ 1 acid solution with 30mL of water, seal the seal and shake slowly until the sample dissolves. Then place it in the dark for 5 minutes. Then titrate to the endpoint with sodium thiothiate standard titration solution.
3.2 Parameters
| Titration mode: | Dynamic titration | ||
| Electrode equilibrium time: 4 seconds | Pre-stirring time: 6 seconds | ||
| Electrode equilibrium potential: 1mv | Titration speed: standard |
| Minimum addition volume: 0.02mL | Pre-titration addition volume | ||
| Final volume: 30mL | pre-titration stirring time: 8 seconds | ||
| Potential spike: 500 | Pre-controlled mv value: none |
4. Results and Discussion
4.1 Experimental Results
Blank volume: 25.943mL
|
Sample name |
Sample weight ( g ) |
Titration volume ( mL ) |
Sodium sulfite content ( % ) |
Average value ( % ) |
|
Wastewater |
0.08956 |
13.285 |
88.71 |
88.71 |
|
0.08711 |
13.631 |
88.72 |
||
|
0.08742 |
13.581 |
88.76 |
||
|
0.09101 |
13.088 |
88.66 |
Calculation formula
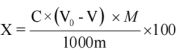
In the formula:
X - The content of sodium sulfite in the sample, with the unit of (%);
C - The concentration of sodium thiosulfate solution, measured in moles per liter (mol/L); V0 - The volume of sodium thiosulfate solution consumed for blank use, measured in milliliters (mL);
V1 - The volume of sodium thiosulfate solution consumed by the sample, in milliliters (mL); M - The molar mass of sodium sulfite, measured in grams per mole (g/mol);
m - The mass of the sample, with the unit of (g);
4.2 Atlas
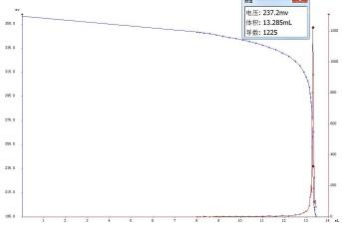
4.3 Conclusion
In this paper, the content of sodium sulfite in wastewater was determined by potentiometric titration. The experimental data show that the T960 potentiometric titrator has good repeatability and can meet the needs of the analysis work.
Precautions
Sodium thiosulfate and iodine solution should be kept away from light.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


