July 11, 2025
Tag:
Biological membranes, such as the cell membranes of the human body or the walls of capillaries, generally have the properties of semi-permeable membranes. The phenomenon where a solvent diffuses from a low-concentration solution to a high-concentration solution through a semi-permeable membrane is called osmosis, and the pressure required to prevent osmosis is called osmotic pressure. Osmotic pressure plays an extremely important role in various biological processes involving the diffusion of solutes or the liquid transport through biofilms. Therefore, when preparing drug preparations such as injections and ocular liquid preparations, attention must be paid to their osmotic pressure. For preparations with osmotic pressure regulators added in the prescription, the osmotic pressure molar concentration should all be controlled.
Preparations such as intravenous infusions, nutritional solutions, electrolytes or osmotic diuretics (such as mannitol injection) should indicate their osmotic pressure molar concentration on the drug instructions, so that clinicians can appropriately handle the preparations used (such as dilution) according to actual needs. The osmotic molar concentration range of normal human blood is 285 to 310mOsmol/kg. The osmotic molar concentration of 0.9% sodium chloride solution or 5% glucose solution is comparable to that of human blood. The osmotic pressure of a solution depends on the number of solute particles in the solution and is one of the number-dependent properties of the solution. It is usually expressed as the Osmolality of osmotic pressure, which reflects the total contribution of various solutes in the solution to the osmotic pressure.
The unit of osmotic pressure molar concentration is usually expressed as the milliosmotic pressure molar of the solute per kilogram of solvent. The milliosmotic pressure molar concentration (mOsmol/kg) can be calculated by the following formula:
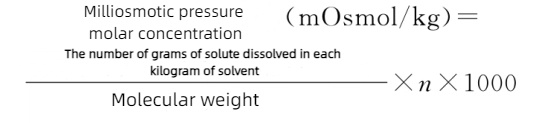
In the formula, n represents the number of particles formed when a solute molecule dissolves or dissociates. In an ideal solution, for instance, glucose n=1, sodium chloride or magnesium sulfate n=2, calcium chloride n=3, and sodium citrate n=4.
In the physiological range and very dilute solutions, the osmotic pressure molar concentration deviates slightly from the calculated value under ideal conditions. As the solution concentration increases, the actual osmotic pressure molar concentration decreases compared with the calculated value. For instance, for 0.9% sodium chloride injection, calculated by the above formula, the molar concentration of milliosmotic pressure is 2×1000×9/58.4=308mOsmol/kg. However, in reality, at this concentration, the n of the sodium chloride solution is slightly less than 2, and the actual measured value is 286 mosmol /kg. This is because under such concentration conditions, the two ions formed by the dissociation of one sodium chloride molecule will undergo a certain degree of association, reducing the number of effective ions. The theoretical osmotic pressure molar concentration of complex mixtures (such as hydrolyzed protein injection solutions) is not easy to calculate, so the actual measured values are usually adopted for expression.
1. Determination of osmotic molar concentration
The osmotic pressure molar concentration of a solution is usually indirectly determined by measuring the drop in its freezing point. In an ideal dilute solution, the freezing point drop follows the relationship ΔTf=Kf·m, where ΔTf represents the freezing point drop, Kf is the freezing point drop constant (1.86 when water is the solvent), and m is the molar concentration by weight. The osmotic pressure conforms to the relationship of Po=Ko·m, where Po is the osmotic pressure, Ko is the osmotic pressure constant, and m is the molar concentration by weight of the solution. Since the concentrations in the two formulas are the same, the osmotic molar concentration of the solution can be determined by the freezing point drop method. The instrument (Recommended instrument: Shanghai Jiahang JH-ST3000 Osmotic pressure Molar Concentration Meter) is designed based on the principle of freezing point drop. It usually consists of a refrigeration system, a thermal sensitive probe for measuring current or potential difference, and an oscillator (or metal probe). When conducting the measurement, immerse the probe in the center of the test solution and lower it into the cooling tank of the instrument. Start the refrigeration system. When the temperature of the test solution drops below the freezing point, the instrument uses an oscillator (or metal probe) to induce the solution to freeze and automatically records the temperature at which the freezing point drops. The measured value displayed by the instrument can be the temperature at which the freezing point drops or the osmotic pressure molar concentration.
The preparation of the standard solution for the calibration of the osmotic molar concentration meter: Take the reference sodium chloride reagent, dry it at 500-650℃ for 40-50 minutes, and then place it in a desiccator (silica gel) to cool to room temperature. According to the requirements, precisely weigh an appropriate amount based on the data listed in Table 1, dissolve it in 1kg of water, shake well, and the result is obtained.
Table 1 Standard Solutions for calibration of osmotic pressure Molar concentration meters
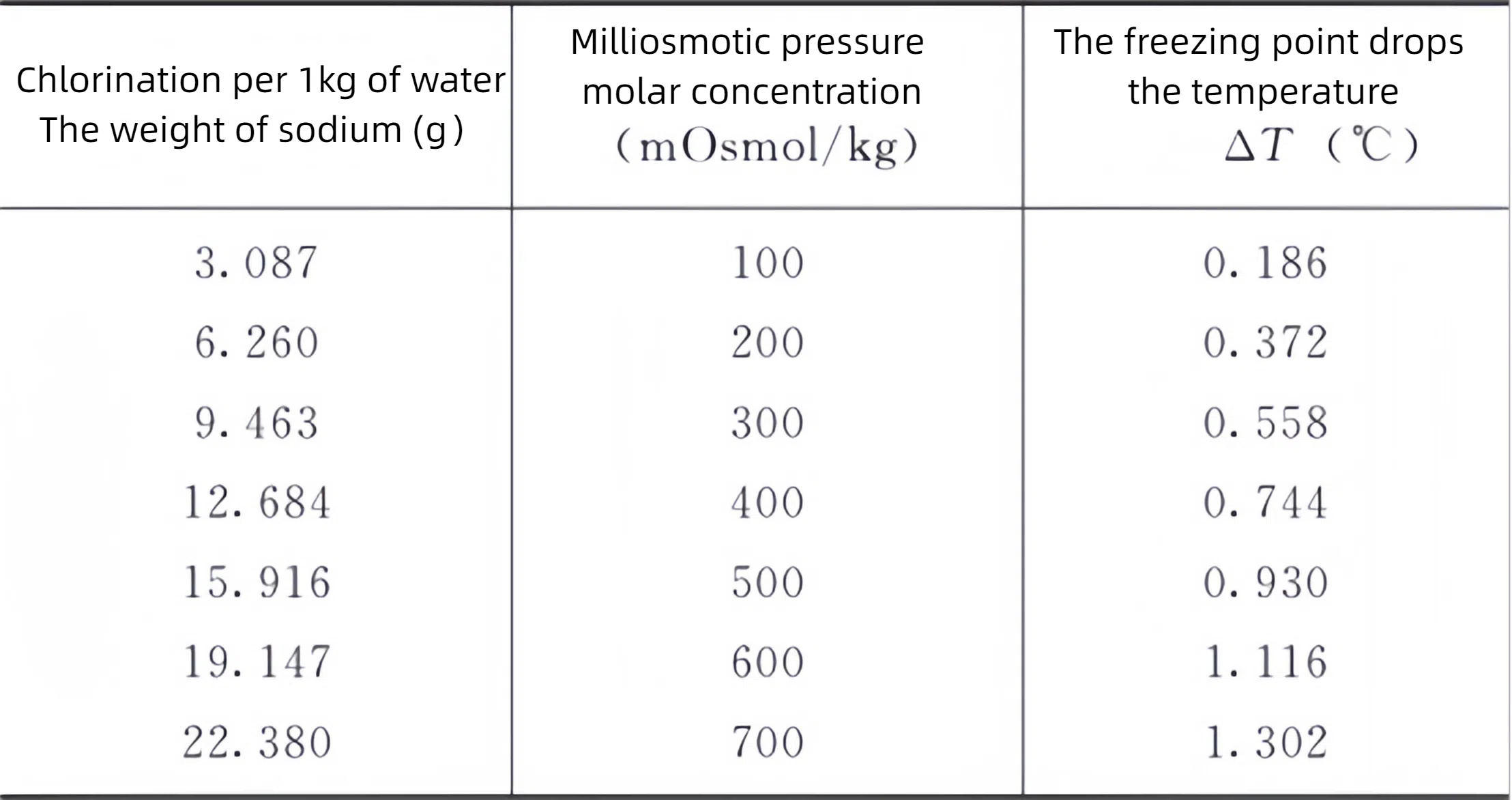
Unless otherwise specified, the test solution should be directly determined in accordance with clinical usage or prepared in accordance with the specific dissolution or dilution methods specified under each variety item, and its molar concentration should be within the determination range listed in the table. For example, sterile powder for injection can be dissolved and diluted in the solvent specified on the drug label or instructions before measurement. It should be particularly noted that after the test solution is diluted, the interaction between particles is different from that of the original solution. Generally, the osmotic pressure molar concentration of the original solution cannot be simply calculated by multiplying the diluted measured value by the dilution factor.
The determination method should be operated in accordance with the instrument manual. First, take an appropriate amount of freshly boiled and cooled water to adjust the zero point of the instrument. Then, select two standard solutions from Table 1 (the osmotic pressure molar concentration of the test solution should be between the two) to calibrate the instrument. Finally, measure the osmotic pressure molar concentration or the freezing point drop value of the test solution.
2 Determination of osmotic pressure molar concentration ratio
The osmotic pressure molar concentration ratio of the test solution to the 0.9% (g/ml) sodium chloride standard solution is called the osmotic pressure molar concentration ratio. The osmotic pressure molar concentrations OT and OS of the test solution and 0.9% (g/ml) sodium chloride standard solution were determined respectively by an osmotic pressure molar concentration meter. The method was the same as that for the determination of osmotic pressure molar concentration, and the osmotic pressure molar concentration ratio was calculated by the following formula

The preparation of the standard solution for the determination of the molar concentration ratio of osmotic pressure: Take the reference sodium chloride reagent, dry it at 500-650℃ for 40-50 minutes, and place it in a desiccator (silica gel) to cool to room temperature. Take 0.900g, accurately weigh it, dissolve it in water and dilute to 100ml, shake well, and it is ready.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


