July 09, 2025
Tag:
Density refers to the mass of a substance contained in a unit volume at a specified temperature, that is, the ratio of mass to volume. Relative density refers to the ratio of the density of a certain substance to that of water under the same temperature and pressure conditions. Unless otherwise specified, the temperature is 20℃.
The relative density of a pure substance remains a constant under specific conditions. However, if the purity of the substance is insufficient, the measured value of its relative density will change with the variation of purity. Therefore, determining the relative density of a drug can be used to check the purity of the drug.
The relative density of liquid drugs is generally determined by a specific gravity flask (Figure 1). The relative density of volatile liquids can be determined by a Vickers specific gravity scale (Figure 2). The relative density of liquid drugs can also be determined by the oscillating hydrometer method.
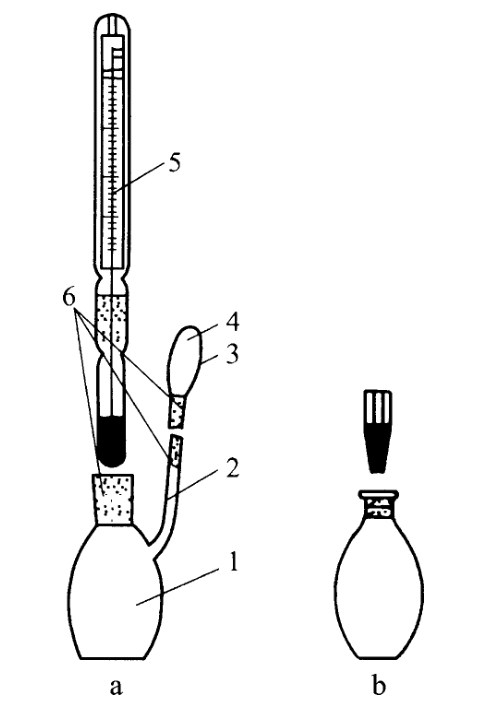
Figure 1 specific gravity bottle
1. The main body of the specific gravity bottle 2 Side tube 3 Side hole 4 Cover 5 Thermometer 6 Glass grinding edge
When using a specific gravity flask for determination (referring to the placement environment of the specific gravity flask and the balance), the temperature should be slightly lower than 20℃ or the temperature specified under each variety item.
1. Specific gravity bottle method
(1) Take a clean, dry and precisely weighed specific gravity bottle (Figure 1a), fill it with the test sample (the temperature should be below 20℃ or the temperature specified under each variety), install a thermometer (there should be no air bubbles in the bottle), and place it in a water bath at 20℃ (or the temperature specified under each variety) for several minutes Make the temperature of the contents reach 20℃ (or the temperature specified under each variety item), remove the liquid that overflows from the side tube with filter paper, and immediately cover the hood. Then take the specific gravity bottle out of the water bath, wipe the outside of the specific gravity bottle clean with filter paper, accurately weigh it, subtract the weight of the specific gravity bottle to obtain the weight of the test sample, pour out the test sample, clean the specific gravity bottle, fill it with freshly boiled cold water, and measure the weight of water at the same temperature according to the above method. Calculate according to the following formula, and the result is obtained.

(2) Take a clean, dry and precisely weighed specific gravity bottle (Figure 1b), fill it with the test sample (the temperature should be below 20℃ or the temperature specified under each variety item), insert it into a stopper with a capillary hole in the center, dry the liquid that overflows from the stopper hole with filter paper, and place it in a constant temperature water bath at 20℃ (or the temperature specified under each variety item) for several minutes. As the temperature of the test solution rises, excessive liquid will continuously overflow from the stopper hole. At any time, dry the top of the stopper with filter paper. Once the liquid no longer overflows from the stopper hole, quickly take the specific gravity flask out of the water bath and proceed with the determination as described in Method (1) above, starting from "wiping the outside of the specific gravity flask clean with filter paper again", and the result is obtained.
2 Webster's specific gravity scale method
Take a Wechsler specific gravity scale with a relative density of 1 at 20℃ (Figure 2). Fill the attached glass cylinder with freshly boiled cold water to about eight-tenths full. Place it in a water bath at 20℃ (or the temperature specified under each variety), stir the water inside the glass cylinder, adjust the temperature to 20℃ (or the temperature specified under each variety), and immerse the glass hammer suspended at the end of the scale into the water inside the cylinder. The right end of the scale arm is suspended at 1.0000. Adjust the balance screw at the left end of the scale arm to achieve balance. Then, pour out the water in the glass cylinder, dry it, fill it with the test solution to the same height, and adjust the temperature in the same way. Next, immerse the dried glass hammer into the test solution, adjust the number and position of the upstream weights of the scale arm to achieve balance, and read the value to obtain the relative density of the test sample.
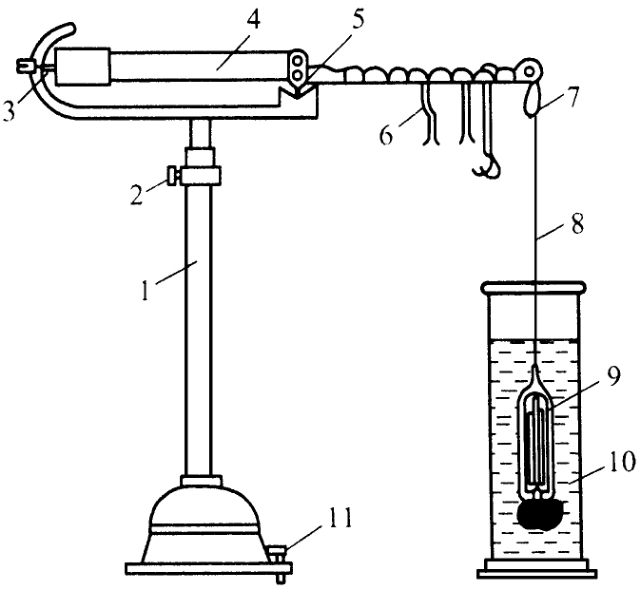
Figure 2 Webster's specific gravity scale
1. Bracket 2 Regulator 3 Pointer 4 Crossbeam 5 Edge 6 Youma 7 Little Hook 8 Fine platinum wire 9 Glass hammer 10 Glass cylinder 11 Adjust the screw
If the relative density of the specific gravity scale is 1 at 4℃, when calibrating with water, the code should be hung at 0.9982, and the relative density of the test sample measured at 20℃ should be divided by 0.9982.
3 Oscillating hydrometer method
The oscillating densitometer mainly consists of a U-shaped oscillating tube (generally made of glass, used for placing samples), an electromagnetic excitation system (to make the oscillating tube oscillate), a frequency counter (used for determining the oscillation period), and a temperature control system. Recommended brand: Shanghai Jiahang AIpol-D70 fully automatic oscillating densitometer.
The density of the liquid sample in the U-shaped oscillation tube can be measured by determining the oscillation period (or frequency) of the sample. The following relationship exists between the oscillation frequency (T) and the density (ρ), the measuring tube constant (c), the mass (M) and volume (V) of the oscillation tube:
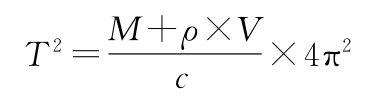
If c/ (4π2×V) is defined as A constant and M/V as B, the above formula can be simplified as follows:
Rho = A * T2 - B
Constants A and B can be determined by adding two substances of known density to the oscillating tube. Commonly used substances are degassed water (such as freshly boiled cold water) and air. Dry air and degassed water (such as freshly boiled cold water) were respectively added to the sample tubes. The vibration periods Ta of the air and Tw of the water were recorded. The density value da of the air was calculated by the following formula:
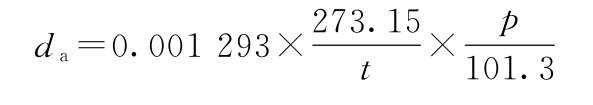
In the formula, da represents the air density at the test temperature, in g/ml;
t is the test temperature, K;
p represents atmospheric pressure, in kPa.
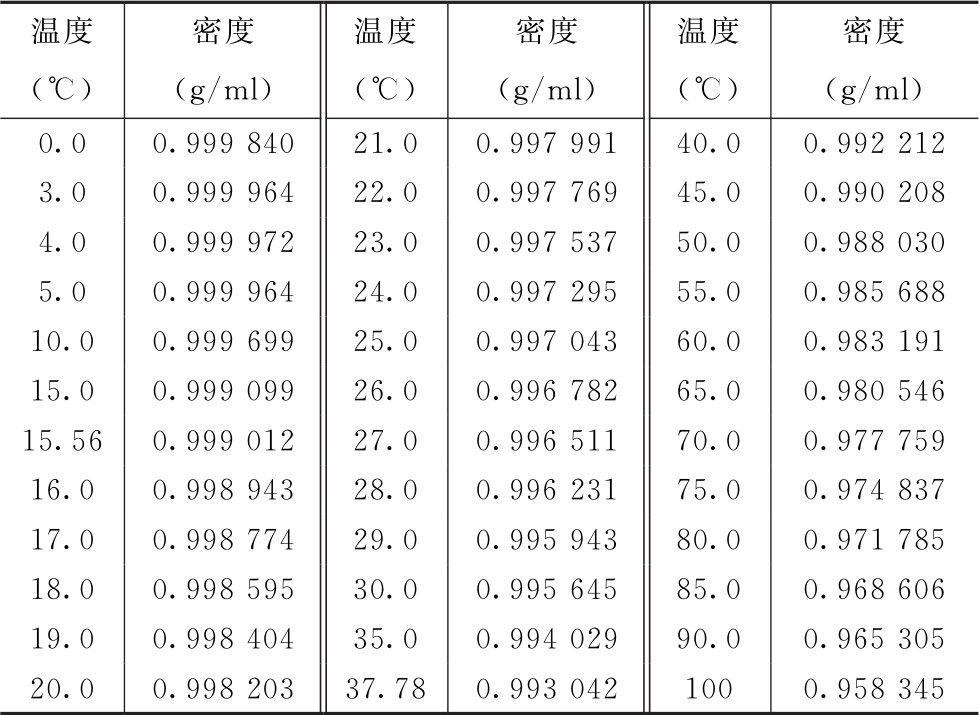
The density value dw of water at the measured temperature is found in Table 1. The constants A and B can be calculated respectively according to the following formulas:
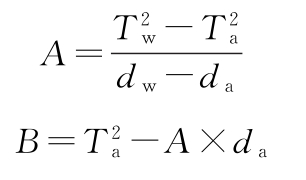
In the formula, Tw represents the oscillation period observed when the sample tube is filled with water, s;
Ta is the oscillation period observed when the sample tube is filled with air, s;
dw is the density of water at the test temperature, in g/ml.
da is the density of air at the test temperature, in g/ml.
If other calibration liquids are used, the corresponding oscillation period T value and d value should be used.
If the instrument has the function of calculating the density from constants A and B as well as the oscillation period measured by the sample, then constants A and B do not need to be calculated. The density value of the test sample can be directly read according to the operation instructions of the instrument manufacturer.
The relative density of substances can be calculated according to the following formula:
Relative density =ρ/0.9982
In the formula, ρ represents the density of the substance being measured at 20℃.
0.9982 is the density of water at 20℃.
The general requirements for the instrument: The reading accuracy of the instrument used for relative density determination should not be less than ±0.001g/ml, and the instrument constant should be calibrated regularly at 20℃ (or the temperature specified in the main text of each variety) using two substances of known density (such as air and water). It is recommended to confirm the accuracy of the instrument's reading with degassed water (such as freshly boiled cold water) before each measurement. The deviation limit can be set according to the instrument's precision. For example, for an instrument accurate to ±0.0001g/ml, the measured value of water should be within the range of 0.9982g/ml±0.0001g/ml. If it exceeds this range, the instrument should be recalibrated.
The determination method is as described in the instrument operation manual. Take the test sample and conduct the determination under the same conditions as when the instrument was calibrated. When measuring, it is necessary to ensure that no air bubbles form in the oscillation tube, and at the same time, it is also necessary to ensure that the actual temperature of the sample is consistent with the measured temperature. If necessary, the temperature of the test sample can be pre-adjusted to approximately 20℃ (or the temperature specified in the main text of each variety) before measurement. This can reduce the risk of bubble formation in the U-shaped shaking tube and shorten the measurement time at the same time.
Viscosity is another important factor affecting measurement accuracy. When conducting the determination of high-viscosity samples, a digital densitometer with viscosity compensation function can be selected for measurement, or a density reference substance similar to the density and viscosity of the test sample (with density within ±5% of the test sample and viscosity within ±50% of the test sample) can be selected to recalibrate the instrument.
Table 1 Density values of water at different temperatures


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


