June 06, 2025
Tag:
1. Preface
DPY dye intermediates are an important component in the dye manufacturing process, including aromatic hydrocarbon derivatives used for the production of dyes and organic pigments. At present, the application of fuel intermediates has expanded to the pharmaceutical industry, pesticide industry, pyrotechnic and explosive industry, information recording materials industry, as well as the production sectors of additives, surfactants, fragrances, plastics, synthetic fibers, etc. In the dye manufacturing process, the selection, content and reaction conditions of dye intermediates determine the performance and application of the final dye.
In this paper, the determination method of the content of DPY dye intermediates was improved by potentiometric titration, and the results were accurate and reliable.
2 Instruments and reagents
2.1 Instruments
JH-T7 fully automatic potentiometric titrator, double platinum electrodes.
2.2 Reagents
Potassium bromide, 0.1 mol/L sodium nitrite standard titration solution, p-aminobenzenesulfonic acid, hydrochloric acid.
3 Experimental Methods
3.1 Experimental Procedures
Calibration of the standard titration solution: Accurately weigh 0.1g (accurate to 0.0001g) of anhydrous p-aminobenzenesulfonic acid into the titration cup and dissolve it in ammonia water. Add 30mL of ice water and 20mL of hydrochloric acid, and titrate with a standard sodium nitrite titration solution.
Sample testing: Accurately weigh 0.5g (accurate to 0.0001g) of the sample and transfer it to a 250mL beaker; Add 150 ml of distilled water and ice cubes, then add 10 ml of hydrochloric acid and 1g of potassium bromide, and stir at a uniform speed. Titrate with standard sodium nitrite solution.
3.2 Parameter Settings
Calibration parameter Settings for sodium nitrite:
| Titration type: Permanent stop titration | Method name: Sodium nitrite calibration | ||
| Burette volume: 10mL | Sample measurement unit: g | ||
| Working electrode: Double platinum electrode | Reference electrode: None | ||
| Stirring speed: 6. | Stirring speed: 6. | ||
| Sensitivity: 0.01μA | Rapid drop volume: 0.2mL | ||
| Slow drop volume: 0.02mL. | End volume: 10mL | ||
| Terminal current: 100 | Pre-control point: 80 | ||
| nt: 100; Pre-control point: 80 | Result unit: mol/L |
Parameter Settings for titrating DPY dye intermediates with sodium nitrite:
| Titration type: Permanent stop titration | Method name: Sodium nitrite titration DPY | ||
| Burette volume: 10mL | Burette volume: 10mL | ||
| Working electrode: Double platinum electrode | Reference electrode: None | ||
| Stirring speed: 7. | Pre-stirring time: 10 seconds | ||
| Sensitivity: 0.01μA | Rapid drop volume: 0.1mL | ||
| Slow drop volume: 0.02mL | End volume: 10mL | ||
| Terminal current: 30. | Pre-control point: 8 | ||
| Delay time: 30 | Correlation coefficient: 0.287 | ||
| Titrant name: Sodium nitrite | Theoretical concentration: 0.0809 |
4 Results and Discussion
4.1 Experimental Results
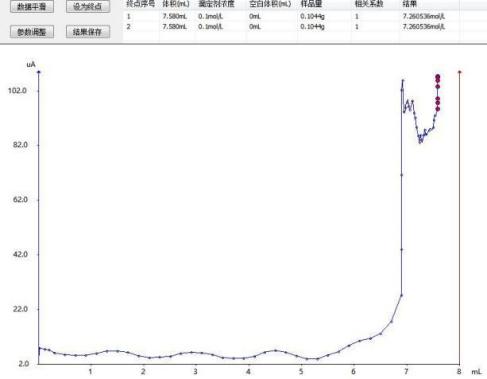
After analysis and testing, the calibration results of sodium nitrite are as follows:
| Sample Name |
Sampling Quantity /g |
Titration Sample volume V1/mL |
Titrate the blank volumeV2/mL |
c(NaNO2)/mol/L |
c(NaNO2)/mol/L |
| P-aminobenzenesulfonic acid |
0.1044 |
7.580 |
0.02 |
0.0795 |
0.0809 |
|
0.0971 |
6.940 |
0.0808 |
|||
|
0.0983 |
6.880 |
0.0825 |
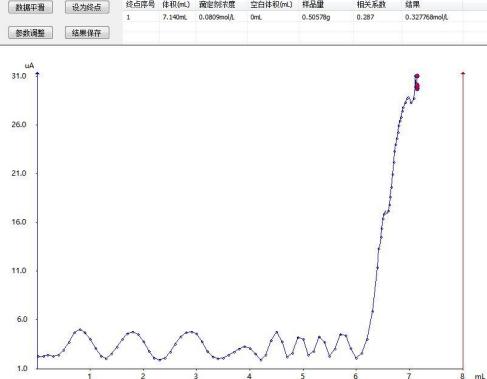
After analysis and testing, the determination results of DPY dye intermediates are as follows:
| Sample Name | Sampling Quantity /g |
c(NaNO2)/mol/L |
Titration Sample volume V1/mL |
Titrate the blank volume V2/mL |
Content /% |
Average value/% |
RSD/% |
|
DPY dye Material intermediate |
0.50578 |
0.0809 |
7.140 |
0.02 |
32.68 |
32.14 |
1.68 |
|
0.52028 |
7.100 |
31.60 |
|||||
|
0.52281 |
7.260 |
32.15 |
4.2 Titration spectrum
Sodium nitrite calibration spectrum
Titration pattern of DPY dye intermediate
4.3 Conclusion
This paper establishes a potentiometric titration determination method for DPY dye intermediates, avoiding errors caused by color determination. The results are more accurate and reliable, providing a certain reference basis for the content determination of DPY dye intermediates.
Precautions
The dosage of hydrochloric acid affects the calibration of sodium nitrite. Reducing the dosage of hydrochloric acid leads to a sudden slowdown in the current, making it difficult to determine the endpoint. It is recommended to add hydrochloric acid dropwise according to the national standard dosage.
2. In several types of permanent stop titration, reversible pair titration cannot achieve pre-control during reversible pair titration, and the reaction endpoint cannot be accurately determined.
When titrating reversible electric pairs with irreversible electric pairs, abnormal spectra will occur, affecting the peak output. Therefore, only reversible pair titration of irreversible pair reactions can be carried out.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


